Primary open-angle glaucoma (POAG) is a type of optic
neuropathy which damages the optic nerve head, causing cupping of the optic disc
and thinning of the neuroretinal rim. These changes result in characteristic
peripheral visual field defects1. High intraocular pressure (IOP) is
regarded as an important risk factor for development and progression of POAG.
Progression of ocular damage can be prevented by lowering the IOP. Different
treatment modalities are available including both surgical and medical options2-4.
The most commonly used drugs for the treatment of POAG are beta-blockers,
carbonic anhydrase inhibitors, alpha-agonists and prostaglandin analogs2,5,6.
Aqueous humor production and IOP levels follow a circadian
rhythm, being high in morning and decreasing by night. Similarly the systemic
blood pressure also decreases during sleep hence affecting the ocular perfusion
pressure. This gives more importance to the nocturnal control of IOP in
management of glaucoma2. Previous research has demonstrated
variability in the effectiveness of topical medications used for IOP control
during different times of the day. This includes decreased effect of
beta-blockers during sleep. Also, acetazolamide and apraclonidine decrease the
rate of aqueous flow at night7-9.
Prostaglandin analogs have been in clinical use in the
management of glaucoma since 19955. Latanoprost reduces the IOP by
increasing the aqueous drainage through the uveoscleral route5,6,10.
Topical prostaglandin analogs generally have no significant systemic side
effects. The adverse effects associated with the use of topical latanoprost are
blurred vision, burning, itching or redness of the eye, changes in eyelash
color and length and pigmentary changes in the iris and periocular skin11-14.
This study was
undertaken to show the effectiveness and safety profile of latanoprost 0.005%
in reducing the intraocular pressure to acceptable levels.
MATERIALS AND METHODS
This prospective non-randomized
open-label study was carried out at the Department of Ophthalmology, Holy
Family Hospital, Rawalpindi from July to December 2015. Fifty patients with
primary open-angle glaucoma (POAG) were enrolled. Following baseline
measurements of IOP, topical latanoprost 0.005% was administered once daily in
the evening for 12 weeks. Patients were followed up with visits at two, six and
twelve weeks. Mean IOP reduction was taken as the primary parameter. The ocular
side effects of the drug were assessed by patient’s history and slit lamp
examination.
Patients of both genders, ≥18
years of age, either newly diagnosed with POAG or those previously diagnosed
but showed progression of disease with topical POAG treatment other than
prostaglandin analogs were enrolled. For new patients, the diagnosis of POAG
was based on IOP value of more than 21 mmHg with optic disc examination
demonstrating glaucomatous damage and/or corresponding visual field defects on
automated perimetry11.
Patients having closed or barely open
anterior chamber angle or those with a history of acute angle closure in the
past were excluded from the study. Also, patients having ocular inflammation or
infection, those who had previously used topical prostaglandin analogues, had
surgical or laser treatment for glaucoma, those using contact lenses or having
any condition preventing applanation tonometry, known hypersensitivity to any
component of the study medication were excluded.
The baseline visit comprised of
complete medical history including treatment history, complete slit lamp
examination, Goldman applanation tonometry, gonioscopy and dilated fundus
examination with special emphasis on the optic disc and peri-papillary area for
typical glaucomatous changes. IOP was measured at 10 am (± 1 hour) and the mean of three readings was noted. Status of
the eyelashes, periocular skin and iris color was also noted. No anterior
segment photographs were taken.
Patients were instructed to instill the
medication in the evening, preferably at 8 pm (± 1 hr). If the patients were already using some other topical
medication for POAG they were instructed to continue it along with latanoprost.
The total duration of treatment was three months (twelve weeks) with follow up
visits at two, six and twelve weeks. A deviation of ± 2 days for first follow up visit (i.e. at 2 weeks) and ± 5 days for second and third visits at six and twelve weeks
was allowed. Patients with adverse events at the end of study period were
followed up for further 2 to 4 weeks. At each follow-up visit a complete
ophthalmic examination including slit lamp examination, IOP measurement and
dilated fundus examination was performed. The adverse effects were diagnosed on
the basis of patient’s history and clinical examination. Severity was graded by
the examiner as mild, moderate or severe. For patients treated for both eyes,
only one randomly selected eye was included for analysis.
The data was
analyzed by Statistical Package for Social Sciences (SPSS) version 20.0 and
values were expressed in terms of frequencies, percentages and means. The mean
IOP reduction was analyzed by comparing mean IOP at follow up visits to the
mean IOP at baseline using Student’s t-test. A P-value of < 0.05 was taken
as significant. The occurrence of side effects was also analyzed and was
expressed in terms of frequencies and percentages.
RESULTS
Fifty patients were enrolled for the study, 22 (44%) were
male and 28 (56%) were female. The age ranged from 28 to 70 years with a mean
of 59.56 (± 9.24) years. Left eye was selected for
analysis in 31 (62%) and right eye in 19 (38%) patients. 40 (80%) patients were
newly diagnosed and 10 (20%) were already using topical IOP reducing
medications other than latanoprost (Table 1, Fig. 1). Table 2 shows the topical
medications used by patients.
The mean baseline IOP was 22.48 mmHg (± 6.4). The IOP at first follow up visit i.e. 2 weeks was
17.72
Table
1: Demographic Distribution of Patients
(n=50).
|
Parameters
|
N (%)
|
|
Gender
|
|
Male
|
22 (44)
|
|
Female
|
28 (56)
|
|
Eyes
|
|
Left
|
31 (62)
|
|
Right
|
19 (38)
|
|
Diagnosis
|
|
New cases
|
40
(80)
|
|
Old cases
|
10
(20)
|
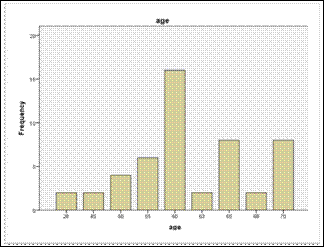
Fig.
1: Age Distribution of Patients (n = 50).
Table 2: Topical Medications Already Being Used
Other Than Latanoprost 0.005% (n = 50).
|
Medication
|
N (%)
|
|
None
|
40
(80)
|
|
Beta Blocker
|
2 (4)
|
|
Beta Blocker + CAH* Inhibitor
Combination
|
8 (16)
|
* CAH- Carbonic Anhydrase
mmHg (± 4.70). This showed a mean reduction of
4.76 mmHg (± 3.26). The second follow up visit at 6
weeks showed a mean IOP of 14.88 mmHg (± 4.19). There was a reduction of 7.60 mmHg (± 4.80) as compared to the baseline IOP. The mean IOP at
final follow up visit at 12 weeks was 13.20 mmHg (± 3.03), showing a mean reduction of 9.28 mmHg (± 5.36) from baseline. A statistically significant reduction
of IOP was seen at 2 weeks as compared to the baseline values (p < 0.001).
This effect continued to increase and at the end of study period there was
marked difference between the baseline and final IOP readings (p < 0.001) (Table
3, Fig. 2). The results showed a 21.17% reduction of IOP from baseline at 2
weeks of treatment with. At 6 weeks there was 33.80% reduction. At 12 weeks, a
mean reduction of 41.28% in IOP was noted (Fig. 3).
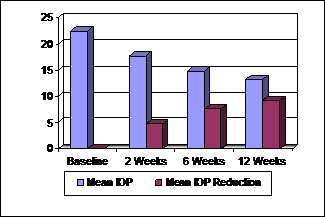
Fig. 2:
IOP Values (n = 50).
A total of 12 (24%) patients had ocular side effects of
medication. 8 (16%) patients reported ocular irritation. Of these, 4 (8%) had
mild symptoms and 4 (8%) had symptoms of moderate degree. None of them required
discontinuation of medication. 2 (4%)
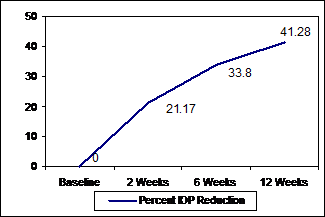
Fig. 3: Percent IOP Reduction.
patients developed conjunctival hyperemia and another 2 (4%)
had watering of eyes after using the drops. Both conjunctival hyperemia and
watering were of mild degree. None of the patients developed any serious
adverse effects. 38 (76%) patients tolerated the medication well and did not
develop any side effects (Table 4, Fig. 4 and 5).
DISCUSSION
As new treatment modalities are being introduced for
glaucoma management, research on the IOP lowering efficacy and safety is
important in clinical decision-making. Numerous
topical medications can be used in glaucoma patients. The six classes of drugs
(miotics, beta-blockers, alpha-agonists, adrenalin derivatives, carbonic
anhydrase inhibitors and prostaglandin analogs) are commonly being used either
as a single drug therapy or in different combinations offering multiple medication
options5,15.
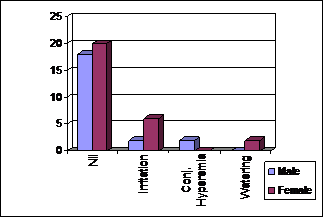
Fig. 4: Adverse Effects According to Gender (n = 50).
This short term study was designed to evaluate the effect
and safety profile of latanoprost 0.005% in controlling the IOP.
Latanoprost was given to patients with inadequate IOP control, and the drugs
already being used by previously diagnosed patients of POAG were not washed out
before adding latanoprost. The results showed a significant decrease in IOP at
two, six and twelve weeks of therapy. The mean IOP reduction at 12 weeks was
9.28 mmHg. Previous literature also showed a similar IOP reduction pattern.
This was achieved in patients who were newly diagnosed with POAG, and were
given only latanoprost for treatment, as well as in patients previously diagnosed
and already using other topical IOP lowering agents16,17.
Fig.
5: Adverse Effects According to Age (n = 50).
This
study did not compare the IOP lowering effect of latanoprost with other topical
drugs or IOP reducing modalities. Several previous studies showed that
latanoprost had a similar, and in some cases better IOP lowering profile as
compared to beta blockers and topical carbonic anhydrase inhibitors2,6,18,19.
Nagar et al. showed that latanoprost had a better effect in controlling IOP as
compared to selective laser trabeculoplasty (SLT)20.
A major concern in glaucoma management
is poor compliance with topical therapy. Most of the drugs usually require
twice or thrice daily administrations. Hence, another advantage of using
latanoprost is better compliance of treatment since it has to be administered
once daily in the evening.
The safety profile of topical latanoprost is excellent. The
reported side effects were only of mild to moderate degree and none of the
patients needed to discontinue the treatment. Previous literature also
demonstrated a similar side effect profile with no serious local or systemic
adverse effects with the use of topical latanoprost1,13,17. In
patients with systemic diseases e.g. heart disease and asthma, bradycardia and
bronchospasm have been reported by the use of topical beta blockers. Therefore,
caution is necessary in treating such patients. Latanoprost has no effect on
the circulatory and respiratory systems and can safely be used in cardiac or
asthmatic patients 18. Eyelash thickening and lengthening, iris and
periocular hyperpigmentation are other reported side effects of long term use
of topical prostaglandin analogs11,17. Since this was a short term
study, so no such adverse effects were seen in any of the patients.
CONCLUSION
It can be concluded that topical latanoprost 0.005% is very
effective in reducing the IOP in glaucomatous patients. It also demonstrates
good pharmacological effects when used along with other drugs that have not
been able to keep the IOP at desired level. It has excellent safety profile
with no significant side effects and offers more convenience to the patients as
a result of once daily administration.
Author’s Affiliation
Dr.
Muhammad Imran Janjua
MBBS,
Postgraduate Trainee Ophthalmology
Dept of Ophthalmology.Holy Family
Hospital, Rawalpindi
Dr.
Saira Bano
MBBS,
Postgraduate Trainee Ophthalmology
Dept of Ophthalmology, Holy Family
Hospital, Rawalpindi
Dr.
Ali Raza
MBBS.
MCPS. FCPS, Head of Ophthalmology Department
Dept
of Ophthalmology, Holy Family Hospital, Rawalpindi
Role of Authors
Dr.
Muhammad Imran Janjua
Study Conception, Data Collection,
Analysis Drafting.
Dr. Saira Bano
Study Conception, Data Collection,
Analysis.
Dr. Ali Raza,
Critical Review, Analysis, Overall
Supervision.
REFERENCES
1. Eveleth D, Starita C, Tressler C. A
4-week, dose-ranging study comparing the efficacy, safety and tolerability of
latanoprost 75, 100 and 125 μg/mL to latanoprost 50 μg/mL (xalatan) in the treatment
of primary open-angle glaucoma and ocular hypertension. BMC Ophthalmology. 2012; 12: 9. Doi:10.1186/1471-2415-12-9.
2. Orzalesi
N, Rossetti L, Invernizzi T, Bottoli A, Autelitano A. Effect of Timolol, Latanoprost, and Dorzolamide on Circadian IOP in
Glaucoma or Ocular Hypertension. Invest
Ophthalmol Vis Sci. 2000; 41: 2566-73.
3. Heijl A, Leske MC, Bengtsson B, Hyman L,
Bengtsson B, Hussein M. Early Manifest Glaucoma Trial Group. Reduction of
intraocular pressure and glaucoma progression: results from the Early Manifest
Glaucoma Trial. Arch Ophthalmol. 2002;
120: 1268-79.
4. The
Advanced Gglaucoma Intervention Study (AGIS). 7. The relationship between
control of intraocular pressure and visual field deterioration. The AGIS
Investigators. Am J Ophthalmol. 2000;
130 (4): 429-440.
5. Pacella
F, Turchetti P, Santamaria V, Impallara D, Smaldone G, Brillante C, et al.
Different Differential activity and clinical utility of latanoprost in glaucoma
and ocular hypertension. Clinical Ophthalmol. 2012; 6: 811–815.
6. Bucci MG. The Italian Latanoprost Study
Group. Intraocular pressure lowering effects of latanoprost monotherapy versus
latanoprost or pilocarpine in combination with timolol: a randomized, observer
masked multicenter study in patients with open angle glaucoma. J Glaucoma,
1999; 8 (1): 24-30.
7. Brubaker RF. Flow of aqueous humor in
humans. Invest Ophthalmol Vis Sci. 1991;
32: 3145–3165.
8. Reiss GR, Lee DA, Topper JE, Brubaker RF.
Aqueous humor flow during sleep. Invest
Ophthalmol. Vis Sci. 1984; 25: 776–778.
9. Topper JE, Brubaker RF. Effects of
timolol, epinephrine, and acetazolamide on aqueous flow during sleep. Invest Ophthalmol Vis Sci. 1985; 26: 1315–1319.
10. Bill A, Phillips CI. Uveoscleral drainage
of aqueous humuor in human eyes. Exp
Eye Res. 1971; 12: 275–281.
11. Kanski
JJ, Bowling B. Clinical
Ophthalmology. 7th Edi. London; Elsevier Saunders, 2011: 384-385.
12. Rowe
JA, Hattenhauer MG, Herman DC. Adverse side effects associated with
latanoprost. Am J Ophth. 1997; 124
(5): 683–685.
13. Alm A, Grierson I, Shields MB. Side
Effects Associated with Prostaglandin Analog Therapy. Survey of Ophth. 2008; 53
(6): S93–S105.
14. Nakakura
S, Yamamoto M, Terao E, Nagatomi N, Matsuo N, Fujisawa Y, et al.
Prostaglandin-associated periorbitopathy in latanoprost users. Clinical Ophthalmology, 2015; 9: 51–56.
15. Alm A, Widengard I, Kjellgren D, Soderstrom
M, Fristrom B, Heijl A, et al. Latanoprost administered once daily caused a
maintained reduction of intraocular pressure in glaucoma patients treated
concomitantly with timolol. Br J Ophthalmol. 1995; 79: 12-16.
16. Mckibbin M, Menage MJ. The effect of
once-daily latanoprost on intraocular pressure and pulsatile ocular blood flow
in normal tension glaucoma. Eye, 1999; 13: 31-34.
17. Thomas R, Parikh R, Sood D, Vijaya L, Sekhar GC, Sood NN,
et al. Efficacy and Safety of Latanoprost for Glaucoma Treatment: A Three-Month
Multicentric Study in India. Indian
J Ophthalmol. 2005; 53: 23-30.
18. Zhang WY, Po ALW, Dua HS, Blanco AA.
Meta-analysis of randomised controlled trials comparing latanoprost with
timolol in the treatment of patients with open angle glaucoma or ocular
hypertension. Br J Ophthalmol. 2001;
85:
983–990.
19. Peeters
A, Schouten JSAG, Severens JL, Hendrikse
F, Prins MH, Webers CAB. Latanoprost versus timolol as first choice therapy
in patients with ocular hypertension. A cost-effectiveness analysis. Acta
Ophthalmol. 2012; 90: 146–154.
20. Nagar
M, Ogunyomade A, O’Brart DPS, Howes F, Marshall J. A randomised,
prospective study comparing selective laser trabeculoplasty with latanoprost
for the control of intraocular pressure in ocular hypertension and open angle
glaucoma. Br J Ophthalmol. 2005; 89: 1413–1417.

![]()



